
These reactions are frequent (and indicate that your body is making. KFF COVID-19 vaccine monitor: April 2021. Common side effects include pain at the injection site, fever, body aches and headaches. Coronavirus (COVID-19) update: FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals. Individuals who have received one or more doses of a monovalent COVID-19. Postmarketing reports: Acute urticaria, delayed urticaria, erythema multiforme. Side effects for the Moderna vaccine when used as a booster include pain or tenderness where you had the injection, swelling of the lymph nodes in the arm where. Individuals 6 years of age and older: Unvaccinated individuals: A single dose of Moderna COVID-19 Vaccine, Bivalent. Postmarketing reports: Myocarditis, pericarditis.
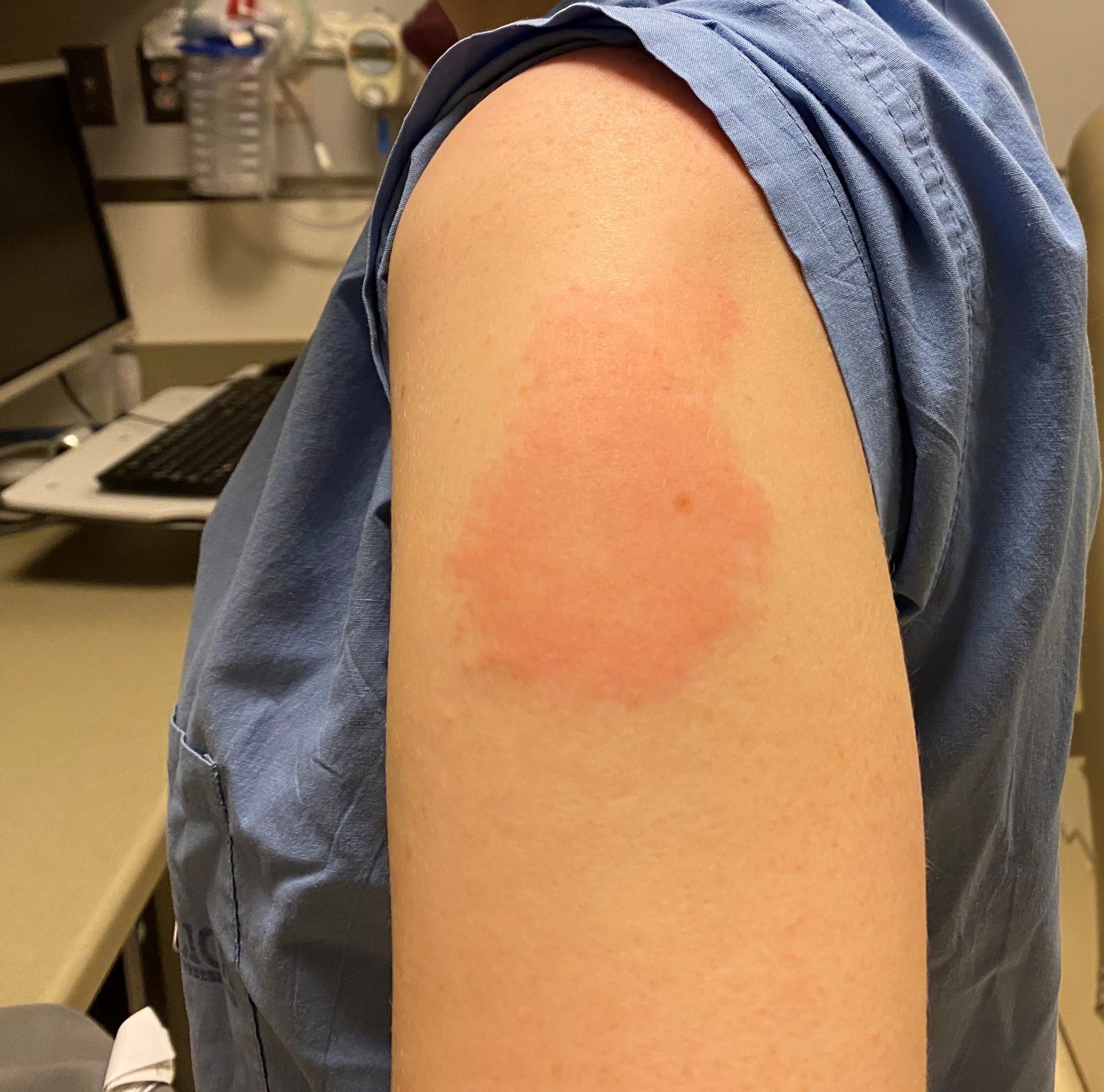
doi:10.1056/NEJMc2108861įood and Drug Administration. The most commonly reported side effects included injection site pain, headache, fatigue, and myalgia. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Possible side effects after getting a COVID-19 vaccine.
MODERNA BOOSTER SIDE EFFECTS SERIES
Janssen COVID-19 vaccine.Ĭenters for Disease Control and Prevention. The most commonly reported side effects by individuals who received a booster dose of the Moderna COVID-19 Vaccine after completion of a two-dose primary series were pain, redness and. Safety monitoring of an additional dose of COVID-19 vaccine - United States, August 12–September 19, 2021. The most common side effects reported after getting a third shot of an mRNA vaccine, the type made by Moderna and Pfizer, were pain at the injection site, fatigue, muscle pain, headache and. CDC recommends additional boosters for certain individuals. CDC endorses ACIP’s updated COVID-19 vaccine recommendations.Ĭenters for Disease Control and Prevention. COVID-19 vaccine boosters.Ĭenters for Disease Control and Prevention. Coronavirus (COVID-19) update: FDA takes multiple actions to expand use of Pfizer-BioNTech COVID-19 vaccine.Ĭenters for Disease Control and Prevention.

Coronavirus (COVID-19) update: FDA expands eligibility for Pfizer-BioNTech COVID-19 vaccine booster dose to children 5 through 11 years.įood and Drug Administration. Allergic reactions Rare reactions that have been reported Ongoing monitoring of side effects and reactions Vaccine safety Only vaccines that meet the safety, effectiveness and quality standards of Health Canada are approved for use in Canada. Coronavirus (COVID-19) update: FDA expands eligibility for Pfizer-BioNTech COVID-19 booster dose to 16- and 17-year-olds.įood and Drug Administration.

COVID-19 vaccines for specific groups of people.įood and Drug Administration. Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines.Ĭenters for Disease Control and Prevention. Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters.įood and Drug Administration.


 0 kommentar(er)
0 kommentar(er)
